A lire sur: http://www.technologyreview.com/news/522996/2013-the-best-biomedicine-stories-of-the-year/
By Susan Young
By Susan Young
While the fights and fumbles over the Affordable Care Act dominated headlines in 2013, the year was also heady with advances in biomedicine. In April, President Obama announced an ambitious federal initiative to map the activity of all the neurons in a brain circuit or, ideally, a whole brain. The $100 million Brain Research through Advancing Innovative Neurotechnologies (BRAIN) project will support neuroscientists, nanotechnologists, and others who propose to develop new technologies that can monitor thousands of neurons simultaneously.
The hope is that such new innovations could help neuroscientists understand the biological origin of cognition and perception and speed the development of treatments for disorders such as autism or post-traumatic stress disorder. There was remarkable progress in the field of neuroscience this year, but researchers still struggle to understand and treat the brain.
This year, the FDA approved the first artificial retina prosthetic for use in the United States following the California-made device’s European approval in 2011. That light-detecting system can replace some of the visual information lost by patients whose retinas, which are an extension of brain tissue, have become damaged by genetic disease. A German company announced that an alternative retina prostheses system had helped patients detect everyday object like doorknobs and read large letters.
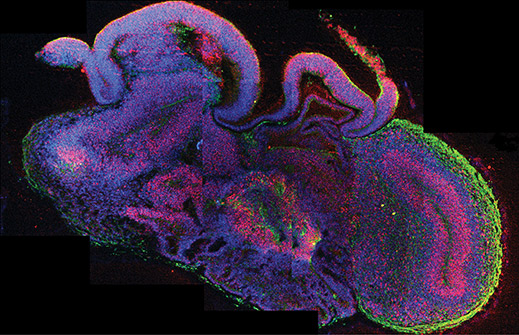
Mini brain: An organoid derived from stem cells contains different brain regions. Green shows neurons and pink/red shows neuronal stem cells.
This year also brought to light a three-dimensional bit of brain tissue, grown from stem cells in a lab, which could be used to study brain function and dysfunction and to potentially screen new medications for toxicity and efficacy. That work builds on top of progress in the field of regenerative biology to grow liver buds and retina tissues in a dish. Scientists also reported in 2013 that they had created a false memory in mice, by manipulating neurons that bear the memory of a place. The work lays new ground for understanding the cell behavior and circuitry that controls memory.
A new type of deep-brain stimulator was implanted into a patient for the first time this year. Deep-brain stimulators are used to deliver therapeutic electric pulses to treat disorders ranging from Parkinson’s to obsessive-compulsive disorder. The new device can also record brain activity, which could one day lead to self-regulating implants and may more immediately give researchers an unprecedented look at brain disorders in patients during day-to-day activities.
Many brain disorders remain difficult to treat. Despite a pressing need and large investments in time and money, an Alzheimer’s treatment continues to evadepharmaceutical companies. And new neuroscience techniques are uncovering what seems to be a pervasive brain injury for soldiers and for adults and children who play high-impact sports such as football. In January, researchers reported they had been able to identify signs of a degenerative brain disorder called chronic traumatic encephalopathy in retired NFL players, the first time the condition could be seen in the brain of a living person.
In addition to its big push for brain technologies, the federal government also made big news in biomedicine this year with the Supreme Court’s decision that “natural” human genes cannot be patented. The ruling was mixed—genes that are identical in sequence to those found in the human genome cannot be patented, but lab-modified versions can. The ruling was made on a suit between Myriad Genetics, a medical diagnostics company, and the Association for Molecular Pathology, a scientific society, over whether Myriad could patent sequences from two genes related to the risk of breast and ovarian cancers. The sequences of these genes are used by Myriad in its tests to determine whether patients are at great risk for developing breast or ovarian cancer.
Early in the year, scientists described some of the privacy risks people may undertake when they allow their genetic information to be used in research studies. The potential for unwanted identification of DNA donors helped influence some of the cautious planning behind the U.K.’s push to become a leader in the genomics industry. The country’s National Health Service will integrate genomic analyses into its hospitals and clinics and plans to share aggregated data with researchers from around the world, but will keep all individual-level data within NHS buildings.
In the U.S., the FDA granted its first approval of a next-generation sequencing technology. The regulatory agency says that the DNA-sequencing machine and reagent producer Illumina can market four of its products as diagnostic devices.
Another big piece of genetics news came from the FDA in late November, when the agency ordered personal genetics company 23andMe to stop selling its genetic analysis test. That at-home test includes interpretations of a customer’s risk of a variety of diseases as well as information about ancestry. The company partially met the FDA’s request by putting its health reports on hiatus, but continues to sell a test that provides uninterpreted DNA data along with analysis of ancestry information.
Researchers developing gene therapies continued to see positive news this year. Although U.S. regulators have not yet followed the European Union’s approval of a gene therapy in late 2012, this year American researchers launched new companies to pursue such validation. And late in the year, a new kind of gene therapy company emerged with plans to turn an exciting advance in molecular biology into a therapy. Pioneers of a breakthrough tool for genome editing called Crispr/Cas came together to build a company that will use the techniques to develop treatments for diseases that cannot be addressed with any other methods.
Here’s hoping that one exciting but tentative bit of news continues from 2013: In March, NIH researchers announced that a two-year-old child infected with HIV at birth may have been cured of the virus. Doctors gave the child standard antiretroviral medications within hours of her birth, and at the age of one, doctors could not find any sign of HIV, despite the fact that she was no longer taking the anti-HIV medications.
Aucun commentaire:
Enregistrer un commentaire